 12-04-2015, 06:13 PM
12-04-2015, 06:13 PM
|
#21
|
|
Registered User
Join Date: Oct 2014
Location: Santa Rosa, CA
Posts: 520
|
I know it may sound like a dumb question but when you change the oil before storage, do you have to make it on the storage spot? Or you can change it at your indy and drive 20 miles back home?
__________________
2003 2.7 Boxster - Tiptronic - Carrera wheels - OBC - Red calipers - Cat pipes - Modified muffler - Rear speakers - K&N - Litronics
2006 V6 Mustang
2008 ML 350
|

|

|
 12-04-2015, 06:27 PM
12-04-2015, 06:27 PM
|
#22
|
|
On the slippery slope
Join Date: Mar 2014
Location: Austin and Palm Springs
Posts: 3,803
|
you need to flatbed it
Just kidding. a short drive won't hurt anything
__________________
2004 Boxster S 6 speed - DRL relay hack, Polaris AutoTop DIY
2004 996 Targa Tip
Instructor - San Diego region
2014 Porsche Performance Driving School
2020 BMW X3, 2013 Ram 1500, 2016 Cmax, 2004 F-150 "Big Red"
|

|

|
 12-04-2015, 08:22 PM
12-04-2015, 08:22 PM
|
#23
|
|
Registered User
Join Date: Oct 2014
Location: Santa Rosa, CA
Posts: 520
|
Quote:
Originally Posted by JayG
you need to flatbed it
Just kidding. a short drive won't hurt anything
|
Thank you sir!
__________________
2003 2.7 Boxster - Tiptronic - Carrera wheels - OBC - Red calipers - Cat pipes - Modified muffler - Rear speakers - K&N - Litronics
2006 V6 Mustang
2008 ML 350
|

|

|
 12-05-2015, 12:30 PM
12-05-2015, 12:30 PM
|
#24
|
|
Registered User
Join Date: Sep 2013
Location: Calgary
Posts: 177
|
JayG is correct - an engine left in dirty oil is a train wreck about to happen.
Used to go with a buddy to old farms looking for old motors.
He would pull the stick - If oil was fairly clean he would make an offer.
If oil was dirty he walked away.
He had no idea of acid build up and we learned of this at a later date.
All he new was if the engine was in dirty oil they were usually trashed in the inside.
When I store anything I put fresh oil in it - Lawn mower, Snow Blower, ect.
Have a great day and to the fella that the mechanic says it can sit in old oil while stored - good luck with that.
|

|

|
 12-06-2015, 07:31 AM
12-06-2015, 07:31 AM
|
#25
|
|
Registered User
Join Date: Nov 2009
Location: Madison, Georgia
Posts: 1,012
|
This is not a diesel dump truck with half a million miles. OP is talking about a 2000 with 57k and less than 1500 miles on the current oil.
But in typical hyperbolic internet forum fashion the comments make it sound like actually using the car before storage will damage the champagne molecules in the magic virgin snake oil and cause the interior of the motor to turn to goo. Also the guru competition has been swift to arrive in this thread. One of my least favorite things about the 986forum. Other sites are not stuck in this dynamic.
That being said...I would proffer that 1500 mile oil that is circulated around the motor from time to time coating said goo inclined parts is far superior to any 29 vestal virgin oil that sits in the sump from December to May.
I started a 993 recently that had not had any special storage measures taken and had not run since 2010 other than being kept in a temp controlled garage and guess what, after the usual precautions and fluid changes it runs like Thor's Hammer. If old oil was as detrimental to the engine internals as is indicated in this thread, Thor would certainly show it.
So if you guys want to argue about how many oil angels can sit on the head of a pin then go ahead. OP: keep the 1500 mile oil and start your car twice a month. It's not the oil I worry about it is the gas that will turn to jelly.
__________________
2001 Boxster S 3.6L, Zeintop
"Calling upon my years of experience, I froze at the controls." - Stirling Moss
Last edited by landrovered; 12-06-2015 at 07:53 AM.
|

|

|
 12-06-2015, 07:53 AM
12-06-2015, 07:53 AM
|
#26
|
|
Registered User
Join Date: Nov 2013
Location: Emerald City
Posts: 885
|
Quote:
Originally Posted by landrovered
This is not a diesel dump truck with half a million miles. OP is talking about a 2000 with 57k and less than 1500 miles on the current oil.
But in typical hyperbolic internet forum fashion the comments make it sound like actually using the car before storage will damage the champagne molecules in the magic virgin snake oil and cause the interior of the motor to turn to goo. Also the guru competition has been swift to arrive in this thread. One of my least favorite things about the 986forum. Other sites are not stuck in this dynamic.
That being said...I would proffer that 1500 mile oil that is circulated around the motor from time to time coating said goo inclined parts is far superior to any 29 vestal virgin oil that sits in the sump from December to May.
I started a 993 recently that had not had any special storage measures taken and had not run since 2010 other than being kept in a temp controlled garage and guess what, after the usual precautions and fluid changes it runs like Thor's Hammer.
So if you guys want to argue about how many oil angels can sit on the head of a pin then go ahead. OP: keep the 1500 mile oil and start your car twice a month. It's not the oil I worry about it is the gas that will turn to jelly.
|
Then long term effects of acidic oil wearing on my original ims bearing which is sitting in it all winter are why I find it necessary to change my oil before and after storage. This is best practice.
If it was Joe Gibbs oil that was costing me a fortune I might rethink my procedure. But since I get my Castrol edge fairly inexpensively it's a no brainer to change it because I like my car and I like the peace of mind. Oh and also the consensus from experienced porsche mechanics is that you should change your oil before storage.
|

|

|
 12-06-2015, 09:34 AM
12-06-2015, 09:34 AM
|
#27
|
|
Registered User
Join Date: Jan 2014
Location: Texas
Posts: 221
|
This is fun. And funny! What about gear oil in the transmission? Sounds like it should be changed twice a year as well - lots of metal, oil and heat. My personal favorite is the slap to the poster with "only 10 posts". Is it the consensus that he's an idiot, cause with only 10 posts, how could he know anything? Now I fear I'll be shamed due to lack of posts. What is the minimum to be one of the "guys" here? I feel like a victim of Lackofpostsism, and we need the DOJ on this stat!
__________________
2000 Boxster S
2010 Volvo XC60
2011 GMC Denali HD 6.6L (sold)
2008 Cayenne S (sold)
1989 Targa (sold)
|

|

|
 12-06-2015, 09:34 AM
12-06-2015, 09:34 AM
|
#28
|
|
Registered User
Join Date: Nov 2009
Location: Madison, Georgia
Posts: 1,012
|
Quote:
Originally Posted by jdraupp
Then long term effects of acidic oil wearing on my original ims bearing which is sitting in it all winter are why I find it necessary to change my oil before and after storage. This is best practice.
If it was Joe Gibbs oil that was costing me a fortune I might rethink my procedure. But since I get my Castrol edge fairly inexpensively it's a no brainer to change it because I like my car and I like the peace of mind. Oh and also the consensus from experienced porsche mechanics is that you should change your oil before storage.
|
If 1500 miles will create acidic oil then why do we not drain this caustic poison from our engines all together? And this is not 1500 miles on jiffy mart 99cent oil, this is full synthetic oil which once upon a time many manufacturers specified a 15,000 mile service interval. We don't drain it though, we run it and coat the insiders of our engines with it so that it can protect the metal from friction and RUST.
Sitting engines lock up because the pistons seize to the cylinder walls and water from condensation from temperature changes causes rust in the bearings, valve train, chains etc. To properly store an engine you mist the cylinders with oil to prevent this, no one has mentioned this practice in this thread but hey, what do I know.
Once again, starting or simply turning the engine over to circulate oil will do far more to protect your engine than anything else. I would not throw out 1500 mile oil if I were the OP.
You guys can do whatever you like, they are your cars and I don't fault people for erring on the side of caution but I think it can get silly when you throw perfectly good oil away twice a year without testing it. If the data says its old then it's old, otherwise it is a waste of money.
__________________
2001 Boxster S 3.6L, Zeintop
"Calling upon my years of experience, I froze at the controls." - Stirling Moss
|

|

|
 12-06-2015, 11:06 AM
12-06-2015, 11:06 AM
|
#29
|
|
Registered User
Join Date: Nov 2013
Location: Emerald City
Posts: 885
|
Quote:
Originally Posted by landrovered
this is full synthetic oil which once upon a time many manufacturers specified a 15,000 mile service.
|
And they were wrong.
Quote:
Originally Posted by landrovered
Once again, starting or simply turning the engine over to circulate oil will do far more to protect your engine than anything else. I would not throw out 1500 mile oil if I were the OP.
|
This may be correct if you can run the engine up to temperature and get a good 20 minute run cycle. Otherwise you do more damage to start it than its worth. And as the reason I'm storing my box is due to inclement weather...so that's not feasible. Again, I get what you're saying here, but you haven't convinced me that my regimen is worth altering.
|

|

|
 12-06-2015, 11:09 AM
12-06-2015, 11:09 AM
|
#30
|
|
Registered User
Join Date: Nov 2013
Location: Emerald City
Posts: 885
|
Quote:
Originally Posted by morgal48
This is fun. And funny! What about gear oil in the transmission? Sounds like it should be changed twice a year as well - lots of metal, oil and heat. My personal favorite is the slap to the poster with "only 10 posts". Is it the consensus that he's an idiot, cause with only 10 posts, how could he know anything? Now I fear I'll be shamed due to lack of posts. What is the minimum to be one of the "guys" here? I feel like a victim of Lackofpostsism, and we need the DOJ on this stat!
|
JFP in PA is one of the most knowledgeable porsche experts on here. So if someone shows up to this forum and starts to question his well researched thoughts and experience with no solid information, yeah, that makes that person seem like an idiot.
|

|

|
 12-06-2015, 11:46 AM
12-06-2015, 11:46 AM
|
#31
|
|
Motorist & Coffee Drinker
Join Date: Jul 2014
Location: Oklahoma
Posts: 3,955
|
Quote:
Originally Posted by PatM
...I'm interested in opinions from some of the oil experts, and others.
Thanks for your input
Pat M
|
Mission accomplished.
Tip: Oil, IMS bearings, domesticated furry creatures that climb on cars, and guns involve deeply held beliefs and stir emotions on this forum. Best to just search the closed threads and pick your own religion.
__________________
I am not an attorney, mechanic, or member of the clergy. Following any advice given in my posts is done at your own peril.
|

|

|
 12-06-2015, 12:08 PM
12-06-2015, 12:08 PM
|
#32
|
|
Registered User
Join Date: Nov 2009
Location: Madison, Georgia
Posts: 1,012
|
Quote:
Originally Posted by jdraupp
And they were wrong.
|
Actually not, given proper filtration synthetic oil can last almost indefinitely in terms of viscosity, the problem is the additives break down and no longer perform their intended function.
Quote:
|
This may be correct if you can run the engine up to temperature and get a good 20 minute run cycle. Otherwise you do more damage to start it than its worth. And as the reason I'm storing my box is due to inclement weather...so that's not feasible. Again, I get what you're saying here, but you haven't convinced me that my regimen is worth altering.
|
This entire thread is confusing the function of oil in a stored engine. A running engine needs to be protected from thermal breakdown of the oil which creates varnish and other compounds to form. Detergents and additives are added to decrease the effects of shear and reduce deposits from thermal breakdown amoungst other things.
In a stored engine rust prohibition is the primary function of oil. It does not need to provide lubrication to reduce friction as there is none, it does not face thermal breakdown from high temperatures, it can however be subject to waxes forming and crystallization of certain compounds at lower temps.
This thread has talked about acidic compounds forming in oil. Motor oil has a ph of 6, gasoline is an alkane which is inert and thus has a ph of 7. So if you were to pour gasoline into your motor oil it would not make it acidic, it would make it more basic so the acidic problem referred to earlier is a non sequitur.
I submit that coating an engine internally with a kerosene and paraffin mix prior to
storage would do a better job than the most expensive royal purple if the Royal purple simply sat in the sump.
Look at the old jeeps that were shipped in crates and coated with cosmoline. The US army knew how to prevent rust and corrosion.
And JFP is a smart fellow, so smart he does not need you guys to tell everyone how smart he is for him.
__________________
2001 Boxster S 3.6L, Zeintop
"Calling upon my years of experience, I froze at the controls." - Stirling Moss
Last edited by landrovered; 12-06-2015 at 12:12 PM.
|

|

|
 12-06-2015, 12:40 PM
12-06-2015, 12:40 PM
|
#33
|
|
Registered User
Join Date: Nov 2013
Location: Emerald City
Posts: 885
|
Thanks for the info. I'm off to drain my oil and dump fresh kerosene in.
|

|

|
 12-06-2015, 12:55 PM
12-06-2015, 12:55 PM
|
#34
|
|
Registered User
Join Date: Feb 2005
Location: It's a kind of magic.....
Posts: 6,657
|
Oil break down and ultimately the formation of organic acids (primarily carboxylic acids) occurs due to both moisture and fuel entrainment in the oil. The moisture acts upon the metal salts that are commonly used in motor oils as stabilizers and anti wear additives, cleaving the salt molecule and allowing oxygen (from both entrained air and the molecular oxygen found in both the additive package components and the fuel molecules themselves) to attach to the paraffinic oil chains, ultimately creating aldehydes, ketones, hydro peroxides and carboxylic acids. It is not just the pH of the contaminants.
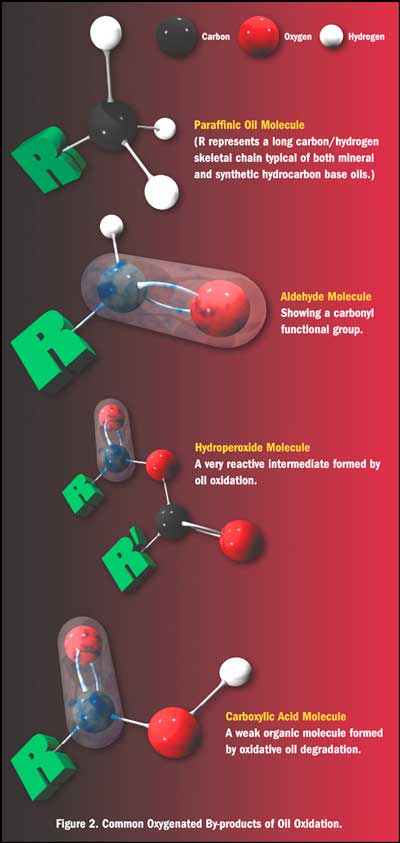
__________________
“Anything really new is invented only in one’s youth. Later, one becomes more experienced, more famous – and more stupid.” - Albert Einstein
Last edited by JFP in PA; 12-06-2015 at 01:07 PM.
|

|

|
 12-06-2015, 01:33 PM
12-06-2015, 01:33 PM
|
#35
|
|
Registered User
Join Date: Nov 2009
Location: Madison, Georgia
Posts: 1,012
|
"Rust inside an assembled engine or transmission can occur any time the oil is allowed to drain off a component due to infrequent use. Engines which are operated daily or weekly seldom encounter this problem, but many street rods, muscle cars, and race cars are often stored for several months without being turned over or fired up. This is a recipe for rusting problems.
Water and low temperatures significantly increase the propensity to rust. Engines fired up infrequently generate a tremendous amount of condensation. If the engine isn’t allowed to completely warm up, this condensation remains inside the engine. (Water will not burn off until the internal engine temperature (oil temperature) reaches 212 degrees F.) This water will then attack any surface which isn’t adequately protected by either an oil film or a vapor phase rust inhibitor ( new tools which often contain a packet of vapor phase rust inhibitor to prevent rusting in shipping and storage)."
Driven Racing Oil Website.
It would appear we have a consensus on water and fuel in the oil as being the problem. As I stated correctly, running the engine periodically to temperature is key in preventing rust.
__________________
2001 Boxster S 3.6L, Zeintop
"Calling upon my years of experience, I froze at the controls." - Stirling Moss
Last edited by landrovered; 12-06-2015 at 01:35 PM.
|

|

|
 12-06-2015, 01:36 PM
12-06-2015, 01:36 PM
|
#36
|
|
Registered User
Join Date: Jan 2014
Location: Texas
Posts: 221
|
Quote:
Originally Posted by jdraupp
JFP in PA is one of the most knowledgeable porsche experts on here. So if someone shows up to this forum and starts to question his well researched thoughts and experience with no solid information, yeah, that makes that person seem like an idiot.
|
The Copernican response. Understood. I shall remain silent. No questioning or opinions.
__________________
2000 Boxster S
2010 Volvo XC60
2011 GMC Denali HD 6.6L (sold)
2008 Cayenne S (sold)
1989 Targa (sold)
|

|

|
 12-06-2015, 01:46 PM
12-06-2015, 01:46 PM
|
#37
|
|
Registered User
Join Date: Feb 2005
Location: It's a kind of magic.....
Posts: 6,657
|
Quote:
Originally Posted by landrovered
[I
As I stated correctly, running the engine periodically to temperature is key in preventing rust.
|
Actually, because such short runs only serve to accelerate the contamination of the oil, entrain more air, and heat the oil up; this only serves to accelerate the oxidation chain I described above, worsening the formation of the organic acids.
We store several high end cars for select clients in a secure environmentally controlled storage facility. None of the car are started for the entire storage period. Over the 30 or so years we have offered this service, UOA's taken in the spring have shown no degradation of the oil, and bore scope cameras have shown no apparent rust formation of any rust or corrosion.
__________________
“Anything really new is invented only in one’s youth. Later, one becomes more experienced, more famous – and more stupid.” - Albert Einstein
|

|

|
 12-06-2015, 02:03 PM
12-06-2015, 02:03 PM
|
#38
|
|
Registered User
Join Date: Nov 2009
Location: Madison, Georgia
Posts: 1,012
|
Are you pulling the plugs and fogging the cylinders with oil before storage?
Also yours is a bit of a trick answer in that the stricter the temperature control is the less formation of condensation there is inside the engine. Also long term storage involves desiccant packs to remove moisture from the air which encapsulates the vehicle in a plastic bags or pods.
Secondly the oil may as well be in a can if it just sits in the sump, It will not degrade if it is kept at a constant temp and humidity and the surface area is limited to the atmosphere.
So remove moisture and keep a constant temp and you can store organic compounds for thousands of years (mummies) so given that a car is a piece of cake.
In a normal garage without humidity control and a non heated area, I would absolutely start my car and allow it to come to temperature every two weeks.
Call me crazy.
__________________
2001 Boxster S 3.6L, Zeintop
"Calling upon my years of experience, I froze at the controls." - Stirling Moss
|

|

|
 12-06-2015, 02:18 PM
12-06-2015, 02:18 PM
|
#39
|
|
Registered User
Join Date: Sep 2014
Location: TN
Posts: 56
|
to change oil or not
I changed the oil today and am sending a sample to Blackstone labs.
I have mentioned my concerns about acid buildup and we will see what they say.
I believe that I will change every 6 months no matter what they say...less than 5000 miles. One of the problems with where I live is winters can be mild or not and with climate change. who knows???? I might put 100 miles a week or maybe 0 a month
Better to be safe than sorry. I surly can't afford an engine......
Thanks again for the input
|

|

|
 12-06-2015, 02:26 PM
12-06-2015, 02:26 PM
|
#40
|
|
Registered User
Join Date: Feb 2005
Location: It's a kind of magic.....
Posts: 6,657
|
OK, you're crazy.
We do not pull plugs or fog engines unless the car is going to be stored for very prolonged periods (1-2 years or more without running). The cars we store are detailed, serviced, filled with StaBil dosed fuel, moved to the storage facility, covered with flannel sheets to prevent the accumulation of dust, and connected to Ctek maintainers. Ambient temperatures inside the facility are held at 55-60F and around 40% RH through the winter. No plastic bags, no desiccants. And then they sit for as much as 5 months.
We actually had a Turbo in storage for a customer, who is a Marine officer, that happened to be deployed to Afghanistan while the car was stored. Unfortunately, he was wounded on his tour, and we ended up keeping the car for him for well over a year before he was able to recover it. Because of how long the car sat, we went over it carefully before returning it to him. It started with the first turn of the key after the dust covers were removed, and a complete check out at the shop revealed with was fine, and still is to this day.
The moisture you need to worry about is the moisture (and fuel) that gets into the oil during normal running, and starts the oxidation and ultimate degradation of the oil. The more miles and heat cycles the oil has seen, the higher the levels of aldehydes, ketones, hydro peroxides and carboxylic acid formation. The process is cumulative, the more run time the oil has seen, the more of these compounds you will find in the oil, which is why you should change it before putting the car up.
__________________
“Anything really new is invented only in one’s youth. Later, one becomes more experienced, more famous – and more stupid.” - Albert Einstein
|

|

|
 Posting Rules
Posting Rules
|
You may not post new threads
You may not post replies
You may not post attachments
You may not edit your posts
HTML code is On
|
|
|
All times are GMT -8. The time now is 04:47 AM.
| |